Ivowen attends EudraVigilance Training Course on Reporting of ICSRs/SUSARs in the EEA – Suspected Adverse Reactions to Medicines
What is EudraVigilance?
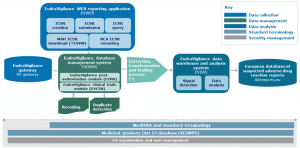
EudraVigilance (http://eudravigilance.ema.europa.eu) is a centralised European database of suspected adverse reactions to medicines that are authorised or being studied in clinical trials in the European Economic Area (EEA) and supports the safe and effective use of medicines (Figure 1 – EudraVigilance System Components). Adverse reactions (ADRs) can be reported during the development and following the marketing authorisation of medicinal products in the EEA. The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network.
ICSR, SUSAR and Safety Signals
Specifically, EudraVigilance is a system for managing and analysing information on suspected adverse reactions to medicines by facilitating electronic exchange of adverse event Individual Case Safety Reports (ICSRs) between EMA and all stakeholders including national competent authorities (NCAs), marketing authorisation holders (MAHs) and sponsors of clinical trials in the EEA. Within clinical trials such a case is referred to as a SUSAR (a Suspected Unexpected Serious Adverse Reaction). The reporting of ICSRs allows for the early detection and evaluation of possible safety signals leading to improved product information and benefit-risk balance for marketed medicines authorised in the EEA. A Safety Signal is defined as information on a new or known adverse event that is potentially caused by a medicine and that warrants further investigation. Signals are generated from several sources such as spontaneous reports, clinical studies and the scientific literature.
Taking into account the pharmacovigilance activities in the pre- and post-authorisation phase, EudraVigilance provides two reporting modules:
- The EudraVigilance Post-Authorisation Module (EVPM)
- The EudraVigilance Clinical Trial Module (EVCTM)
Figure 1. EudraVigilance System Components
Data Publishing, Review and PRAC Evaluation
EMA publishes data from EudraVigilance in the European database for suspected adverse drug reaction reports – http://www.adrreports.eu/. EMA, NCAs and MAHs are responsible for reviewing EudraVigilance data to detect safety signals. The Pharmacovigilance Risk Assessment Committee (PRAC) evaluates the safety signals detected in EudraVigilance and may recommend regulatory action as a result – http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/general_content_000537.jsp&mid=WC0b01ac058058cb18.
What we can do for you:
Electronic reporting of suspected adverse reactions to medicines is mandatory for MAHs and sponsors for clinical trials. Ivowen can advise and assist you in the following areas of reporting to EudraVigilance along with updating product information for PRAC recommendations:
- Describe the Registration process with EudraVigilance
- Create, validate and send safety messages (initial, follow-up reports, nullification reports, literature reports, parent-child , study reports, reports with medical and drug history)
- Create and send acknowledgments of received ICSR messages
- Query, view, browse and download safety reports
- Query, view and browse MedDRA through the EVWEB
Please contact us for further information.
Written by Laura Oakey.