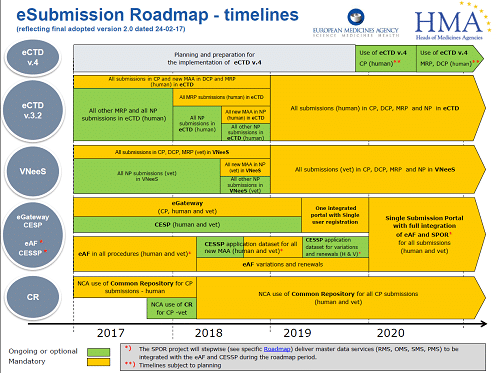
Falsified Medical Directive (FMD) and updated QRD template released February 2016
Falsified medicines are fake medicines that are designed to mimic real medicines. Due to the increase of falsified medicines on the market the EU has a strong legal framework for licensing, manufacturing and distribution of medicines. In July 2011 DIRECTIVE 2011/62/EU (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:174:0074:0087:EN:PDF ) came into force; this directive aims to prevent falsified medicines entering the legal supply chain and thus reaching patients. One of the Directive’s measures is the introduction of safety features on medicines.
Update on Safety Features on Medicines:
On February 9th 2016 the EC published an implementation plan for the introduction of the safety features on the packaging of nationally authorised medicinal products for human use:
In conjunction, a Regulation was also published laying down detailed rules for the safety features appearing on the packaging of medicinal products for human use:
http://ec.europa.eu/health/files/eudralex/vol-1/reg_2016_161/reg_2016_161_en.pdf
What do you need to do?:
The Delegated Regulation will apply in all European countries from the 9th February 2019 (3 years after its publication). Belgium, Greece and Italy have the option of deferring the application of the rules by an additional period of up to 6 years.
There are two safety features to be placed on the packaging of most prescription medicines and certain non-prescription medicines no later than 9 February 2019.
-1). a unique identifier (a 2-dimension barcode) and
-2). an anti-tampering device (ATD).
How does this affect your medicinal products and applications?
New MAAs submitted from April 2016:
- QRD:
- Revised QRD template.
- Revised dossier sections:
- In the case of medicinal products where the ATD is placed on the immediate packaging because there is no outer packaging and the ATD affects the container and its closure system(s), applicants are required to include information on the ATD and how the ATD affects the container and its closure system(s) (sections 3.2.P.2.4 and/or 3.2.P.7 of the Notice to Applicants Volume 2B)
Ongoing MAAs
- QRD:
- CHMP opinion in March 2016 advised to comply Revised QRD template
- CHMP opinion from April 2016 onwards, applicants must comply with the revised QRD template
- Revised dossier sections:
- As per new MAAs
Existing MAs
- QRD:
- Revised QRD template within 3 years – Can be implemented in Type IA, Type IB, Type II, Renewals, Line extension etc. where the submission affects the product information (PI). Approval of submissions must be no later than the 9th February 2019. If no regulatory procedure occurs within the required timeframe a notification is requested to be submitted pursuant to article 61(3).
- Revised dossier sections:
- If the ATD is placed on the immediate packaging and the ATD affects the container and its closure system(s), applicants are required to submit the appropriate variations to include the information on the ATD and how the ATD affects the container and its closure system(s) (see section B.II.e of the Variation Guidelines).
- If the ATD does not affect the container and its closure system, or is placed on the outer packaging, no regulatory procedure is necessary. However, if the addition of the ATD has an impact on the readability of the packaging information, MAHs are requested to submit mock-ups as per http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004891.pdf
Medicinal product no longer needs to bear safety features
- QRD:
- Regulatory procedure to remove the standard statements regarding the unique identifier and ATD.
- Revised dossier sections:
- ATD on immediate packaging: Regulatory procedure to remove the statements regarding the ATD in the dossier. ATD on outer packaging – no regulatory procedure necessary.
Change of Legal Status
- QRD:
- Non-prescription to prescription following a MAH switch application: MAH should use the regulatory procedure to comply with the revised QRD and regulations. Non-prescription to prescription following a Commission referral or a PSUR assessment, the Commission Decision will cover, inter alia, the regulatory requirements to implement the safety features.
- Revised dossier sections:
- Non-prescription to prescription: MAH should use the regulatory procedure to include the information on the ATD and how the ATD affects the container and its closure system(s)
What does the new QRD template now include?
The new QRD template includes the following sections in: Particulars to appear on <the outer packaging> <and> <the Immediate packaging:
- UNIQUE IDENTIFIER – 2D BARCODE
- UNIQUE IDENTIFIER – HUMAN READABLE DATA
Updated QRD template in track changes is available here:
http://www.ema.europa.eu/docs/en_GB/document_library/Template_or_form/2009/12/WC500029823.pdf
Are any medicinal products exempt from the above?
Yes, the medicinal products exempt from the above are listed in Annex I of the Regulation located here:
http://ec.europa.eu/health/files/eudralex/vol-1/reg_2016_161/reg_2016_161_en.pdf
Are any medicinal products not subject to prescription but that should include the safety features above?
Yes, the medicinal products not subject to prescription that shall bear the safety features, referred to in Article 45(2) are listed in Annex II of the Regulation located here:
http://ec.europa.eu/health/files/eudralex/vol-1/reg_2016_161/reg_2016_161_en.pdf
If you have any queries on the above, if you would like any help with complying with the new regulation or if you have any other queries please contact us .
Written by Emily Fletcher.